The coldest places on earth; Ultra Cold Atoms
- Michelle Ncube
- Oct 3, 2025
- 6 min read
Updated: Nov 23, 2025
Have you ever wondered where the coldest places in the universe may be? Surprisingly, they are not in space but in the very laboratories here on Earth. Utilising advanced techniques, physicists can cool atoms up to billionths of a degree above absolute zero. At these extreme temperatures, atoms behave more strangely than ever allowing us to directly observe quantum behaviour that we do not see on a day-to-day basis.
This blog dives briefly into what ultra cooling entails and some the uses of ultra cold atoms in physics.
What is ultra cooling?
Ultra cooling refers to the process of reducing the temperature of a gas of atoms to extremely cold levels. This is usually in the range of micro to nano kelvins above absolute zero, allowing for them to act as quantum simulators which can enable the study of new phases of matter.
Absolute zero, defined as 0K or -273.15 °C, is the lowest theoretical temperature any system can be. This is theoretical due to the notion surrounding systems at absolute zero. In one of my older blogs I covered why it’s a limit and what it really entails. [click here]
Methodology to cool atoms
There are many methods that scientists use to cool atoms. One popular method is by using lasers and other effects such as Doppler and evaporative cooling. But first it is vital to understand the concepts behind how lasers can bring about cooling effects.
Key concept - Lasers
Laser which is an abbreviation of light amplification by stimulated emission of radiation is a device that produces a concentrated beam of light. The light emitted consists of photons with the same energy as one another.
Whenever light hits a boundary, surface or particle, it exerts a force. This is because it has momentum.
Initially this may seem counterintuitive as how can a light have momentum if ρ=mv and photons are massless?
However, ρ=mv is an approximation for objects moving at speeds much slower than light so does not apply to massless particles like photons.
Instead, the full energy-momentum equation is used:

As the intrinsic rest mass of a photon is zero, this equation can be simplified to E = pc showing that light has momentum.
So, when light is incident on an atom, there is a transfer of momentum from the photon to the atom, creating an impulse. As there are trillions of photons from a laser beam transferring momentum per second, this means that there is a rate of change of momentum, allowing a force to be exerted on the atom.
With this knowledge, scientists use 6 laser beams pointing in both the negative & positive directions of x, y & z. This allows for lights’ cooling effects to be experienced by the atoms in 3D, which helps speed up the cooling effects.
Atoms and energy levels
Atoms have quantised energy levels, meaning if a photons energy is similar or matches the energy between the two quantised levels, it is more likely to be absorbed. When this happens, the atom gains momentum in a given direction. As systems tend towards their equilibrium, this photon will then be re-emmited but in a random direction.
Because this emission is random, the momentum kicks from emission average out to zero. However, as photons exert a force on atoms, the absorption of a photon will always push the atom in the direction of the incoming laser beam. This causes a net momentum change which decreases the speed on the atom overtime.
This concept alongside Doppler cooling & evaporative cooling is used to cool atoms down in the labs. Scientists actually use 6 laser beams pointing in both the negative & positive directions of x, y & z . This allows for lights’ cooling effects to be experienced by the atoms in 3D, but for the purpose of this report, I will only explain as if the atom is being cooled in one direction.
Stage 1: Doppler cooling
Scientists first use doppler cooling to bring atoms down from room temperature to about 100micro-kelvin.
Doppler effect
The Doppler effect is the change in frequency of a wave caused by the relative motion between the source of the wave and the observer.

Picture yourself standing still on the side of a road. A few seconds later, you see an ambulance driving past with its siren blasting loudly. As the ambulance moves towards you, the siren gets louder and louder. This is because the wavefront are “bunched up”, this reduces the wavelength. The shorter wavelength means a higher frequency as V = f λ therefore, a higher pitch is observed. As the ambulance moves further away from you, the wavefronts get further apart, increasing the wavelength and decreasing the frequency, resulting in a lower pitch heard. This is the Doppler effect.
Applying the Doppler effect to cooling atoms
As mentioned earlier, atoms have quantised energy levels meaning for an atom to absorb a photon, the photons energy must match the difference in energy levels in the atoms. This means that each atom has a natural resonance frequency. If the laser used to cool the atom has a frequency that is too high or low, the atom will not absorb it.
Scientists tune laser light to a frequency that is slightly lower than the atoms resonance frequency. This makes the laser red tuned, so stationary atoms do not absorb the photons.
If the atom is moving directly towards the laser beam however, the wavefronts will appear to be compressed from the atoms perspective. This shifts the light to a slightly higher frequency which mean the faster the atom moves, the more likely the light can become exactly the resonance frequency. Now the atom can absorb the photon, whilst other atoms moving slower don’t absorb it as they see the light to be red shifted. This allows for selective absorption as in ultra cooling, a group of atoms are cooled down. So, Doppler cooling allows for the fastest atoms to be targeted and cooled which reduces the average kinetic energy (i.e the temperature)
As the atoms absorb photons in one direction and emit the photons in random directions, this causes the net momentum change to be negative, leading to the atoms speed to decrease over time.
Stage 2 : Sub Doppler – Cooling
As the atoms get slower, this causes the light to not be as red shifted as before, preventing absorption meaning there is a limit to which the atom can be cooled. Therefore, other methods must be used to achieve even lower temperatures.
Sub doppler cooling involves cooling atoms below the doppler limit, this is what gets the atoms to micro/nano kelvin range.
A popular method is Sisyphus cooling which stems from the character Sisyphus in Greek mythology.
Atoms repeatedly climb “hills” of light induced potential energy. As an atom moves up one of these hills, it loses kinetic energy each time. At the top of the hill, the atom gets optically pumped back to its original state, causing it to “climb up the hill” again, causing it to lose kinetic energy. This is how it becomes colder.
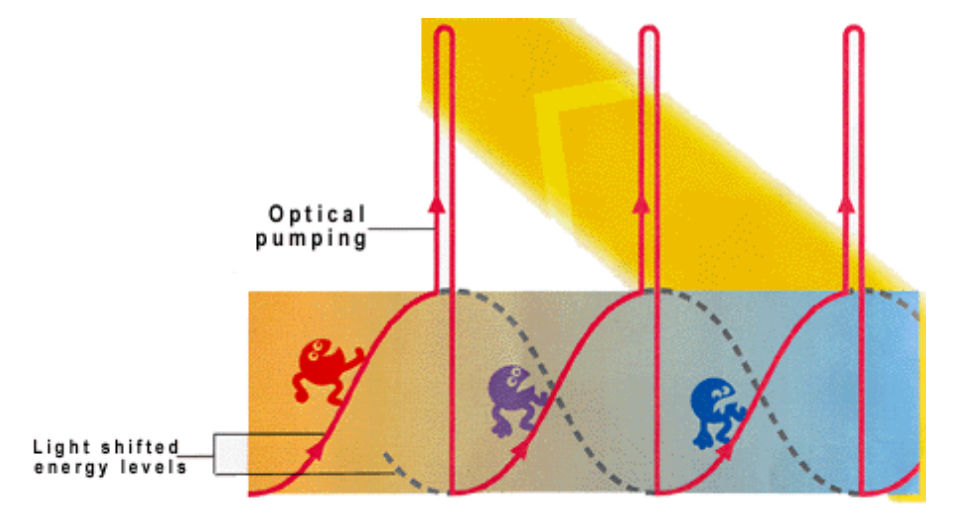
A popular method is Sisyphus cooling which stems from the character Sisyphus in Greek mythology.
Atoms repeatedly climb “hills” of light induced potential energy. As an atom moves up one of these hills, it loses kinetic energy each time. At the top of the hill, the atom gets optically pumped back to its original state, causing it to “climb up the hill” again, causing it to lose kinetic energy. This is how it becomes colder.
Stage 3 : Evaporative Cooling
Evaporative cooling reduces the thermal energy of the system by removing the most energetic atoms. What happens is, the atoms are placed in a magnetic or optical trap. A magnetic trap is a device that uses a magnetic field to confine and manipulate particles whilst an optical trap manipulates light to trap particles. The depth of the trap is then set, which alters the minimum amount of kinetic energy needed to escape the trap. Therefore, the atoms with kinetic energy similar to this move towards the edge of the trap and eventually escape.
This reduces the average kinetic energy as there are less energetic atoms in the trap, therefore reducing the temperature. If the depth decreases, this will reduce the minimum kinetic energy needed to escape, eventually cooling the atoms to fractions above absolute zero.
Applications of ultra-cold atoms in physics/real world:
Now having gained an insight to how ultra cold atoms are cooled; below are some of its applications within physics:
Bose Einstein Condensates
Superfluidity
Analogue black holes
Quantum technology and computing
I’ll be exploring more of these in the upcoming blogs!
Further reading
If you found this topic interesting, try searching up the following and see what comes up:
Magnetic traps and they they work?
What are optical traps?
The different methods in sub Doppler cooling
Sources





Comments